We usually recommend MOG/CFA-induced EAE as a first pass screen for potential MS treatments, even if your drug's exact mode of action is not understood. Its short duration (~25 to 30 days) and use of C57BL/6 mice makes it the least expensive EAE model. It has a large therapeutic window, good sensitivity, and very good predictive value for efficacy in MS. It is also very robust; 10 to 12 mice/group give good statistical results.
This model may also be used to test neuronal regeneration. FTY720 (fingolimod, Gilenya), BG-12 (dimethyl fumarate, DMF, Tecfidera), diroximel fumarate (Vumerity) and methylprednisolone are the most commonly used positive controls.
In this direct EAE model, disease is induced by immunization with MOG peptide or protein.
Most often MOG35-55 peptide antigen is used, but the model can also be run with whole MOG protein (MOG1-125). The disease induced by either antigen is similar, except that EAE induced by whole MOG protein is reported to be dependent on B cells; this may be of interest if your compound targets B cells.
Prophylactic treatment
The results below are from a representative study performed at Hooke. C57BL/6 mice were immunized with MOG35-55 emulsified in CFA and treated daily (QD), p.o., with vehicle or FTY720. Treatment was from Day 0 (day of immunization) through Day 28.
| Vehicle | FTY720 | |
|---|---|---|
| n (# of mice) | 12 | 12 |
| EAE incidence (%) | 100.0% | 41.7% |
| p value (EAE incidence) | - | <0.001 |
| Median day of onset | 13.0 | >28.0* |
| Maximum score | 3.38 ± 0.68 | 1.00 ± 1.35 |
| p value (maximum score) | - | <0.001 |
| End score | 2.83 ± 0.83 | 0.63 ± 0.96 |
| p value (end score) | - | <0.001 |
| Relative end body weight (%) | 91.8% ± 6.3% | 104.1% ± 6.1% |
| p value (body weight) | - | <0.001 |
*Median day of onset cannot be calculated if ≤50% of mice developed disease.
Maximum and end scores compared using Wilcoxon's non-parametric test.
Relative end body weights (%) compared using 2-tailed Student's t-test.
Values are shown as mean ± SD unless otherwise indicated.
Therapeutic treatment
The results below are from representative studies performed at Hooke. C57BL/6 mice were immunized with MOG35-55 emulsified in CFA and treated daily (QD), p.o., with vehicle or FTY720 starting on the 1st day of disease for each mouse through Day 33 (top row) or Day 35 (bottom row).
| Vehicle | FTY720 | |
|---|---|---|
| n (# of mice) | 10 | 10 |
| Day of onset | 11.8 ± 1.0 | 11.9 ± 0.9 |
| Score at enrollment | 0.95 ± 0.64 | 0.95 ± 0.72 |
| Maximum score | 3.35 ± 0.24 | 3.25 ± 0.26 |
| p value (maximum score) | - | 0.374 |
| End score | 2.90 ± 0.46 | 2.25 ± 0.49 |
| p value (end score) | - | 0.011 |
| Relative end body weight (%) | 85.3% ± 7.5% | 93.4% ± 5.5% |
| p value (body weight) | - | 0.013 |
Maximum and end scores compared using Wilcoxon's non-parametric test.
Relative end body weights (%) compared using 2-tailed Student's t-test.
Values are shown as mean ± SD unless otherwise indicated.
| Vehicle | FTY720 | |
|---|---|---|
| n (# of mice) | 10 | 10 |
| Day of onset | 12.8 ± 0.8 | 12.8 ± 0.8 |
| Score at enrollment | 0.85 ± 0.13 | 0.85 ± 0.17 |
| Maximum score | 3.00 ± 0.24 | 2.35 ± 0.58 |
| p value (maximum score) | - | 0.010 |
| End score | 2.55 ± 0.44 | 1.25 ± 0.63 |
| p value (end score) | - | <0.001 |
| Relative end body weight (%) | 97.7% ± 1.7% | 107.4% ± 2.8% |
| p value (body weight) | - | 0.008 |
Maximum and end scores compared using Wilcoxon's non-parametric test.
Relative end body weights (%) compared using 2-tailed Student's t-test.
Values are shown as mean ± SD unless otherwise indicated.
As shown above, the mean maximum score (MMS) of the FTY720 group in therapeutic studies is not always significantly lower than in the Vehicle group, since mice may reach their maximum EAE scores before FTY720 can reduce EAE severity. FTY720 typically takes more than 4 days to reduce EAE score, and the peak of EAE is expected to occur 2 to 5 days after EAE onset.
Chronic EAE develops in C57BL/6 mice after immunization with an emulsion of MOG (either MOG35-55 or MOG1-125) in CFA, followed by administration of pertussis toxin (PTX) in PBS. The emulsion provides antigen which initiates expansion and differentiation of MOG-specific autoimmune T cells. PTX enhances EAE development by providing additional adjuvant and is believed to facilitate entrance of autoimmune T cells into the CNS.
Typically, more than 90% of mice develop EAE 9 to 16 days after immunization. The first wave of EAE usually lasts 7 days, followed by partial recovery and continuing chronic paralysis.
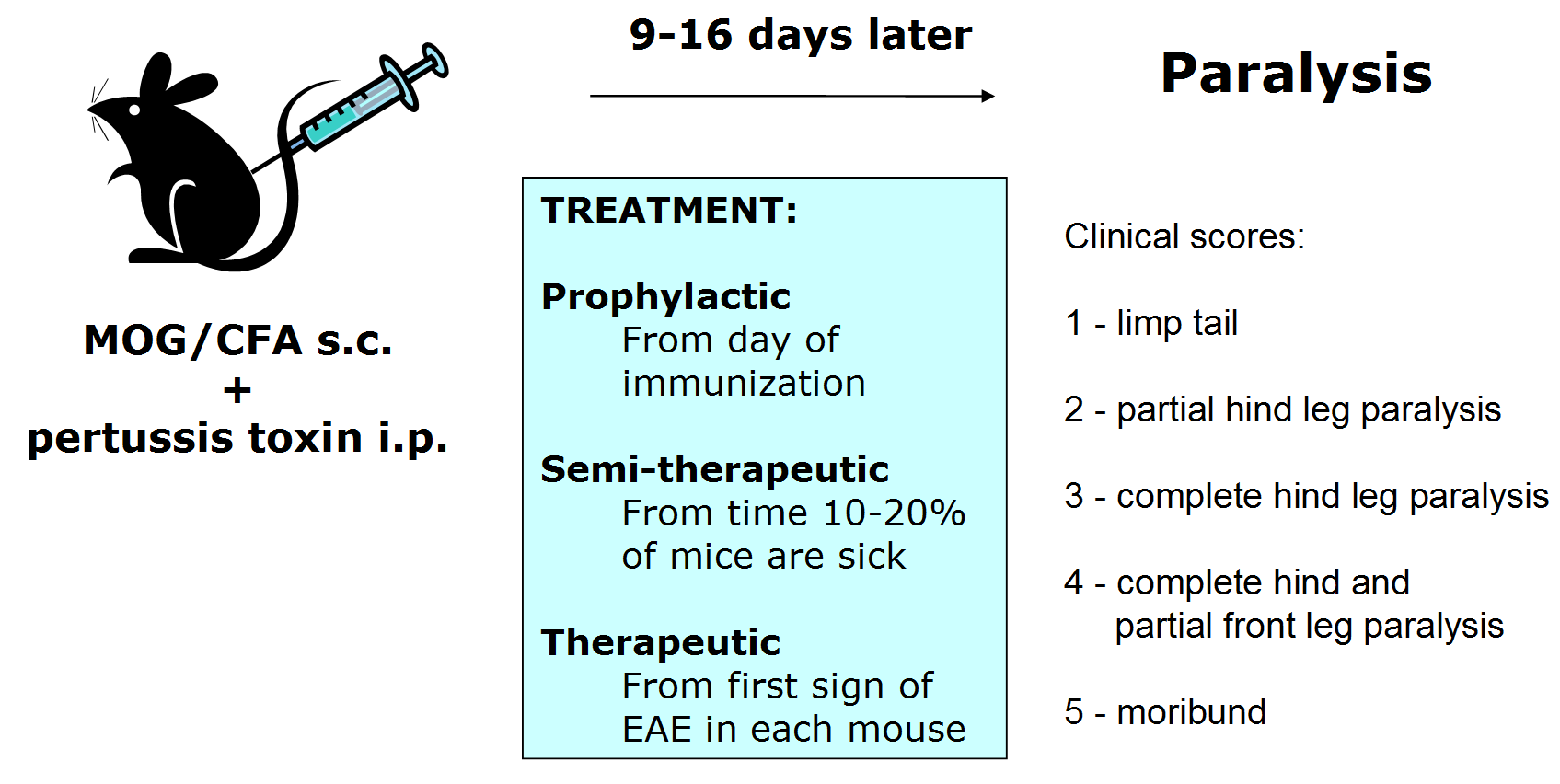
This model can be used with any of the standard EAE treatment regimens.
When used with prophylactic treatment, EAE induced by MOG35-55/CFA in C57BL/6 mice has the largest therapeutic window and is sensitive to the broadest range of targets of any EAE model. We recommend it as an initial screen for compounds aimed at MS treatment.
In prophylactic studies treatment normally lasts 28 days. Median time to disease onset is usually the most sensitive measure of compound efficacy. Small changes in immune response can delay EAE - suppression of antigen presentation or of T cell activation, proliferation, or differentiation into Th1 and/or Th17 cells all delay disease onset.
In addition to FTY720 (fingolimod, Gilenya), methylprednisolone, dexamethasone, glatiramer acetate (Copaxone) and dimethyl fumarate (DMF, BG-12, Tecfidera) are all efficacious when dosed prophylactically in this model.
Overall treatment efficacy is indicated by both delayed onset of EAE and reduced maximum severity (vs. negative controls). Of these, mean maximum score (MMS) is usually more important - reduced MMS indicates an overall reduction in EAE severity.
Semi-therapeutic treatment starts after immunization but before all mice are sick (usually 10 to 11 days after immunization). This is a compromise between prophylactic and therapeutic treatment.
Therapeutic treatment specifically demonstrates whether a compound will reverse the course of disease and/or improve recovery. Therapeutic treatment requires a relatively small amount of drug, because in most studies each mouse receives only 14 days of treatment. We usually recommend this stringent treatment regimen as a follow-up to prophylactic studies, to establish compound efficacy after disease onset.
Rolling enrollment is used - mice are assigned to groups one at a time as they show the first signs of EAE.
To achieve tight results (highly uniform groups), groups are balanced for similar day of EAE onset and similar EAE scores at enrollment; these correlate well with maximum EAE score. Disease is induced in ~15% more mice than will be enrolled, which provides a larger pool of mice for balancing groups.
Treatment of each mouse begins immediately at enrollment. The final day of treatment differs for individual mice depending on their day of enrollment.
Results are usually analyzed by synchronizing scores to the day of disease onset for each mouse.
The most important result in therapeutic treatment is the average clinical EAE score at the end of the study.
Late therapeutic treatment is similar to therapeutic treatment, except that treatment begins a fixed number of days after each mouse shows the first signs of disease. Most often, this treatment regimen is used to evaluate neuroregenerative effects.
In addition to day of EAE onset and onset score, groups are also balanced for maximum score before enrollment.
Hooke offers an extensive set of tissue collection and analysis options. Click here for more information.