
Recommended protocol for use with:
1 EK-0120 was called "PLP139-151/CFA Emulsion" prior to May 2020. Only the name has changed, to avoid confusion with EK-0230.
Note: For kit EK-2120, this protocol version is for use only with Hooke PTX lot number 1011. (Other lots may require a different PTX dose.)
In this protocol "PLP139-151", without further qualification, refers to either [Ser140]-PLP139-151 or native mouse PLP139-151 peptide, as chosen by the investigator.
These kits are recommended for study of relapsing-remitting experimental autoimmune encephalomyelitis (EAE), including testing efficacy of potential therapeutics.
Each kit consists of:
EAE is induced in SJL mice by immunization with an emulsion of PLP139-151 in complete Freund's adjuvant (CFA). When using EK-2120, the initial immunization is followed by administration of pertussis toxin (PTX) in PBS on the day of immunization.
Most mice develop relapsing-remitting EAE with disease onset 9 to 14 days after immunization including PTX (EK-2120), or 10 to 15 days after immunization without PTX (EK-0120 or EK-0230). Peak of disease generally occurs 1 to 2 days after disease onset for each mouse, and lasts 1 to 3 days. Most mice completely or partially recover within 10 days. Usually 50 to 80% of mice will show an increase in EAE severity (relapse) after initial partial or complete recovery 20 to 40 days after immunization.
For study of only the first wave of EAE, we recommend 10 to 12 mice/group.
For study of EAE relapses, we recommend 15 to 20 mice/group. This relatively large number of mice is recommended to ensure that enough mice will relapse for good statistical analysis.
Mice are typically observed for 40 to 45 days after immunization. This is sufficient for at least 50% of mice to relapse.
EK-0120 and EK-2120 contain [Ser140]-PLP139-151 antigen.
EK-0230 contains native mouse PLP139-151 antigen ([Cys140]-PLP139-151), which induces more severe EAE.
EK-2120 is supplied with pertussis toxin (PTX); EK-0120 and EK-0230 are not. PTX induces a more severe initial wave of EAE, but it can also reduce the incidence of relapses.
Most labs will find kit EK-0120 suitable for study of either the initial wave of EAE, or for study of EAE relapses.
The table below summarizes the differences between the kits.
EAE induction kits compared (typical results)
| EK-0120 | EK-2120 | EK-0230 | |
|---|---|---|---|
| Antigen | [Ser140]-PLP139-151 | [Ser140]-PLP139-151 | Native mouse PLP139-151 |
| PTX | No | Yes | No |
| EAE onset1 | 11 to 15 days | 9 to 14 days | 10 to 14 days |
| EAE incidence | 90 to 100% | 90 to 100% | 90 to 100% |
| 1st wave severity | + | ++ | +++ |
| Relapse incidence | 50 to 80% | 20 to 50% | 50 to 80% |
1 Days from immunization
PLP139-151 induced EAE in SJL mice is sensitive to small variations in mice and in laboratory environments. (All testing at Hooke is with SJL mice from The Jackson Laboratory, stock #686).
If EAE severity with EK-0120 is insufficient, and the goal is to study the initial wave of EAE, we recommend kit EK-2120. The PTX in EK-2120 induces a more severe initial wave of EAE, but it can also reduce the incidence and severity of relapses.
If EAE severity with EK-0120 is insufficient, and the goal is to study EAE relapses, we recommend EK-0230, which uses the native mouse PLP peptide. This will induce more severe EAE than EK-0120, and will not reduce relapse incidence.
For the most uniform EAE development, use female SJL/J mice at 8 to 9 weeks of age. All mice should be the same age.
Exclude any mice with tail lesions before immunization (see "Troubleshooting" below).
Hooke recommends and uses SJL mice from The Jackson Laboratory, stock #686.
| Qty | Description |
|---|---|
| 1 | Hooke Kit™ [Ser140]-PLP139-151/CFA Emulsion (EK-0120) or Hooke Kit™ [Ser140]-PLP139-151/CFA Emulsion PTX (EK-2120) or Hooke Kit™ PLP139-151 (native)/CFA Emulsion (EK-0230) See "Kit selection" above. |
| 1 | 50 mL sterile polypropylene tube (EK-2120 only) |
| 10 | SJL mice, females, at 8 to 9 weeks old (The Jackson Laboratory strain SJL/J, stock #686) |
| Phosphate buffered saline (PBS; EK-2120 only) (standard formulation, pH 7.4, calcium-free, magnesium-free) |
Acclimate mice for at least 2 weeks prior to immunization.
Administer each mouse with receive adjuvant emulsion s.c. at 4 sites.
The same day, only if using Hooke Kit™ [Ser140]-PLP139-151/CFA Emulsion PTX (EK-2120), administer PTX in PBS, i.p.
Minimize mouse stress at all times (stress inhibits EAE development). See "Troubleshooting", below.
Each EK-2120 kit comes with a vial containing 750 ng of PTX in 15 µL glycerol buffer (50 ng/µL). This PTX must be diluted with cold PBS (2 to 8 °C) for dosing.
For Hooke PTX lot 1011 only we recommend a dosage of 30 ng PTX. This recommendation is the result of Hooke’s in vivo testing of the PTX lot (potency for EAE induction can differ up to 10 fold between PTX lots). Future lots may require different PTX doses.
Prepare PTX solution fresh (within 30 minutes before injection) under sterile conditions in a biosafety cabinet. Administer after immunization with the emulsion component of the kit, the same day.
Important: The same solution of PTX must be administered to all mice. If more than one kit will be used in a study, prepare PTX for the entire study in one pooled batch.
To prepare the PTX solution:
Calculate the required volume of stock PTX in glycerol from the EK-2120 kit(s). Use the formula:
Number of mice * (PTX dose (ng) * 1.2) / 50 = µL
Example: 30 mice * (30 ng * 1.2) / 50 ng/µL = 21.6 µL
(This includes 20% extra PTX to allow for dosing loss.)
Put 0.12 mL cold PBS for each mouse into a single 50 mL sterile polypropylene tube. (The 0.12 mL includes 20% extra PBS to allow for dosing loss.)
Example: 30 mice * 0.12 mL = 3.6 mL PBS into the tube.
Note: This recommended dose is for Hooke PTX lot 1011 only; future lots may require different PTX doses.
Add PTX to PBS in 50 mL tube:




Good subcutaneous injection technique is important for successful EAE induction; the same person should inject all groups.
These procedures are drafted for right-handed injection; reverse if injecting with the left hand.
Anesthetize mouse per local IACUC and veterinary recommendations. Anesthesia is not strictly required but Hooke recommends isoflurane anesthesia for efficient and precise immunization (figures here show anesthetized mice); for tips on restraining and immunizing unanesthetized mice, see our EAE induction protocol for C57BL/6 mice at https://hookelabs.com/protocols/eaeAI_C57BL6.html.
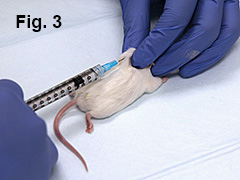
Lay mouse in prone position (belly down). Mouse will be immunized at 4 sites (Fig. 1).
Prime the needle by pushing a small amount of emulsion out onto a sterile tissue and wiping it off (Fig. 2).
With left hand, pinch skin over left shoulder. Using right hand, inject 0.05 mL of emulsion subcutaneously at that site (Fig. 3). Repeat the procedure by injecting 0.05 mL over mouse’s right shoulder.
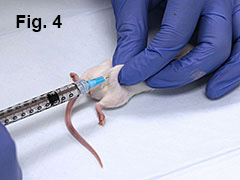
Using a similar technique, inject 0.05 mL of emulsion over mouse’s left and right hip (Fig. 4).
After each injection, keep the needle inserted into the subcutaneous space for 10 to 15 seconds to avoid leakage of the emulsion. Alternatively, a light pull on the syringe plunger will prevent leakage.
Repeat steps 1 to 5 for all mice.
Perform steps 6 to 8 only if using Hooke Kit™ [Ser140]-PLP139-151/CFA Emulsion PTX (EK-2120):
Draw 1 mL PTX solution into 1 mL syringe.
Mount a fresh 27G ½" (12 mm) needle.
Inject each mouse i.p. with 0.1 mL.
Repeat steps 6 to 8 for all mice.
Check mice for signs of EAE daily starting 8 days after immunization. (See Appendix A below.)
As soon as the first signs of paralysis occur, provide mice with food pellets and wet food on the floor of the cage, and HydroGel (ClearH2O, Portland ME) as a source of water. If mice are severely paralyzed (score 3 or greater), inject 1 mL Ringer’s solution s.c., daily or every other day.
All mice will develop obvious bumps of emulsion at the injection sites 2 to 4 days after injection.
In some mice, alopecia will develop at the site of injection 5 to 7 days after injection.
In most mice, the emulsion will remain at the site of injection for the duration of the experiment (typically 40 to 45 days). Approximately 10% of mice will clear the emulsion by developing skin ulcers at injection sites. Most of the time, these ulcers do not require treatment – they usually heal in a few days and scars form. If concerned, consult attending veterinarian. Antibiotic ointment may be applied.
EAE development does not correlate with mice clearing or not clearing emulsion from the injection site.
EAE will be consistently induced in 90 to 100% of mice, with disease onset in most mice occurring 9 to 14 days after immunization including PTX (EK-2120), or 10 to 15 days after immunization without PTX (EK-0120 or EK-0230). The mean maximum score of the first paralytic episode will be between 2.0 and 3.5 (the native PLP139-151 in kit EK-0230 will produce scores toward the higher end of the range).
Even mice that develop a very severe first wave EAE (score 4) will almost always show some recovery within 2 or 3 days; therefore euthanasia due to severe EAE is not recommended in most cases. If mice are unable to reach water due to temporary paralysis, Ringer’s solution should be administered subcutaneously until recovery.
Mean maximum score of the second paralytic episode will be 1.5 to 3.5.
Daily dosing of mice often delays and reduces relapses due to stress of dosing.
The following illustrates typical results (results with undosed mice shown; vehicle-dosed mice will likely develop somewhat less severe EAE and will have lower incidence of relapses due to dosing stress):
In our experience, up to 10% of SJL mice may develop tail lesions for unclear reasons, either before or after immunization (development appears to be unrelated to immunization). Tail lesions appear as an area of raw skin, usually close to the root of the tail.
These may be mild (appears similar to a friction burn) to severe (appearance like a deep wound). If tail lesions develop after enrollment, apply antibiotic ointment immediately. If caught early (when lesions are mild), development of lesions can be reversed.
Mice with severe tail lesions will not develop EAE and should be excluded.
Successful induction of EAE requires low-stress mouse handling and husbandry procedures, good injection technique (as recommended above), use of appropriate mice, and good quality, stable, antigen emulsion.
Stress strongly decreases EAE susceptibility; minimizing mouse stress is very important for successful EAE induction.
For consistent EAE:
Isoflurane anesthesia may be used, and disease often develops better if mice are anesthetized during immunization.
[1] Lyons JA et al, Eur J Immunol 29:3432 (1999)
[2] Mendel I et al, Eur J Immunol 25:1951 (1995)
[3] Thornton V et al, Contemp Top Lab Anim Sci 42:49 (2003)
[4] Marusic S et al, J Exp Med 202:841 (2005)
[5] Thakker P et al, J Immunol 178:2589 (2007)
Because of its many similarities to multiple sclerosis (MS), EAE is used to study pathogenesis of autoimmunity, CNS inflammation, demyelination, cell trafficking and tolerance induction.
EAE is mediated by myelin-specific CD4+ T cells, but CD8+ cells and B cells may also play a role in some models of EAE.
The relapsing-remitting EAE model in SJL mice is the mouse model which most closely resembles human MS.
The model is induced by immunization with [Ser140]-PLP139-151 in CFA emulsion, which may be followed by administration of pertussis toxin (PTX) in PBS if desired to increase severity of the first wave of EAE. The emulsion provides antigen which initiates expansion and differentiation of PLP-specific autoimmune T cells.
PTX enhances EAE development by providing additional adjuvant and facilitating entrance of autoimmune T cells into the CNS. However, in some labs PTX also reduces the incidence and severity of relapses in SJL mice.
This model is usually used to test efficacy of compounds on development of relapses in EAE. It is less often used in a prophylactic treatment study because most mice recover after the first wave of disease, even without treatment.
Most mice develop relapsing-remitting EAE with disease onset 9 to 14 days after immunization including PTX (EK-2120), or 10 to 15 days after immunization without PTX (EK-0120 or EK-0230). The first wave of EAE lasts several days and most mice fully or almost fully recover from it. After a disease-free period, which can last from one day to several months, most mice relapse.
Each individual mouse will have a different course of EAE after the first relapse. Most mice will only partially recover from the first relapse, but some may achieve full recovery. As mice are observed longer, more relapses will develop and less recovery will occur. During the first 5 to 7 weeks after immunization, 50 to 80% of mice will develop a relapse. After several months, almost all mice will have relapsed.
The first wave of paralysis occurs at the same time for all mice in the group, and is associated with a loss of body weight which is mostly regained on recovery from the first wave.
Different mice will experience succeeding waves of EAE at different times (not synchronized). In each wave there is body weight loss at onset (but not as dramatic as in the first wave), most of which is regained at recovery. After the first wave of disease, the average body weight of each group is relatively stable, with normal slow increases with age. This is because individual mice experience relapses at different times, so only a few mice in each group are acutely sick at any given time. Therefore, statistically significant differences in relative body weight between groups are not usually found at the end of the study (typically 40-45 days from immunization).
Both EAE incidence and severity will be reduced if mice are exposed to stress any time after approximately 3 days before immunization. Compound administration is a major source of stress; the more frequent the administration and the less tolerated the vehicle, the greater the reduction of EAE development. Unlike the therapeutic EAE model in C57BL/6 mice, in this model the stress of treatment and administration of vehicle continues to affect disease even after clinical signs of EAE appear.
The most important readouts in this model are incidence of relapse and MMS of the relapse period, calculated for all mice. The MMS of only those mice which relapse rarely shows significant differences between groups because only a fraction of mice relapse and relapse severity tends to be variable even within a single group. The MMS of the relapse period is more sensitive because it is based on all mice in the group and reflects both relapse severity and residual EAE severity in those mice which do not relapse.
Version: 2023-06-04